Intelligent content delivery with Tridion Docs - Key capabilities and advantages of Tridion Docs

Structured content unlocks ROI
In our previous post, “Intelligent Structured Content: How DITA delivers for enterprise content”, we explored how DITA XML and structured content management can transform your enterprise documentation from static files into dynamic, reusable, compliance-ready assets. But structured content alone is just the beginning. Now it's time to delve into how Tridion Docs elevates this further, taking structured content beyond management and into intelligent content delivery, driving significant ROI for your organization.
In this post, we will see the real world techniques, AI-powered search, contextual delivery and automated multichannel publishing, that turn structured content into measurable results. We showcase how Tridion Docs and Tridion Docs Genius leverage AI-driven capabilities like Trustable Chat and Hexahops to create and then deliver content precisely when, where, and how your audiences need it.
Structured content isn't just about better authoring and management, it's about smarter, faster, and more engaging delivery. It's about eliminating the hidden costs of legacy workflows, copy/paste errors, reformatting delays, siloed translations that bleed budget and slow time‑to‑market.
Executives also face a new pressure point
Turning "AI hype" into real productivity gains for key stakeholders, employees and customers. With Tridion Docs, and Tridion Docs Genius, your content becomes an interactive knowledge hub that cuts rework, reduces regulatory risk, and meets modern expectations for instant, AI‑backed answers that your users don't just consult but rely on.
The results? Faster answers, stronger compliance, lower operational costs, and a genuine competitive advantage.
Single source of truth
Tridion Docs manages your content as components, (topics, images, maps), stored in a central repository. This eliminates the chaos of scattered files. Writers and subject matter experts can collaborate on the same library of approved content modules. Everyone from engineering to sales and customer success refence the same facts, improving consistency.
This translates into tangible wins for your organization as a whole:
- Consistency at scale: every channel pulls the same wording, branding and compliance statements so you avoid costly re‑writes and legal risk.
- Single point of change: update once and watch the fix cascade across manuals, portals and chatbots cutting time‑to‑market for updates and new releases.
- Full traceability: audit trails show who changed what, where, why and when as well as pointing to where it is reused. Compliance checks that used to take days shrink to minutes.
- Lower IT overhead: one repository to govern, back up and secure instead of dozens of disconnected file stores. Transferring your content from individual computers to a central server reduces the risk.
For regulated industries, this single-source approach also means enhanced and complete traceability and auditability – you always know where a piece of content is used and can track its revision history, which is crucial for compliance.
Maximum content reuse
Because content is modular in DITA, Tridion Docs helps you reuse content extensively instead of reinventing the wheel. A small team can efficiently maintain a massive documentation set by reusing approved topics across manuals and inserts. For example, HORIBA Medical’s team of just seven writers now manages 13,500+ documents in 15 languages by relying on Tridion’s reuse capabilities. The CCMS ensures that when a source topic is updated, all publications referencing it can be updated or published with confidence.
Waters Corporation uses this model to reuse up to 60 % of its source content, cutting production time and cost.
Read the Waters Corporation case study
Multichannel publishing made easy
Tridion Docs can publish the same DITA content to PDF, HTML5 web, mobile, eLearning, or any format needed, all from the single source. This is critical as businesses strive to deliver content on the channels that customers and regulators demand. With a CCMS, there’s no need to manually recreate content for each output or risk inconsistencies. The result is omnichannel consistency with minimal effort. In regulated domains like medical devices, this means IFUs, online help, and regulatory dossiers all pull from the exact same approved content modules, ensuring 100 per cent alignment across outputs.
Informatica achieved simultaneous shipment (SimShip) of documentation in multiple languages for the first time – publishing English and Portuguese together, rather than waiting 6-9 months to produce the second language.
Informatica removed desktop publishing bottlenecks and saved around USD 6 per page across a 10 000 page set.
Read the Informatica case study
Streamlined Translation and Localization
Translation is often a huge expense and a major risk vector for global companies: when teams translate piecemeal, terminology drifts, updates are missed, and inconsistent messages slip through to customers and regulators. Tridion Docs solves this by giving you one central repository that stores both the approved source content and every language variant. Integrated with RWS translation technology and services, the platform automates hand‑offs, leverages translation memory and tracks linguistic quality, eliminating copy‑and‑paste errors and duplicated spend. Structured reuse reduces the word count before it ever reaches translation, and the single repository means every update ripples out safely to all languages. Tridion Docs is perfectly integrated with translation management systems (a key strength of RWS due to the translation industry standard tool Trados, and decades of translation service experience) to automate content export/import and leverage translation memories (TM).
The impact is dramatic:
Philips Healthcare’s Image Guided Therapy unit was able to translate far more documentation without adding headcount, by connecting Tridion Docs to their translation workflow.
HORIBA Medical, cut translation spending by 50% by eliminating redundant desktop publishing steps and leveraging content reuse for localization.
Faster translation cycles mean faster time-to-market globally.
Seamless flexible integration
Tridion Docs supports extensibility to fit your organization’s needs. Open APIs and standards based architecture let Tridion Docs slot into PLM, eQMS and translation workflows. This integration is essential for medical device and pharma companies facing tight submission deadlines. It natively supports DITA out-of-the-box and can be configured for other schemas if required, offering flexibility for those who might need to accommodate legacy formats like DocBook or even S1000D content in parallel.
Tridion’s architecture allows integrations with other enterprise systems, for example, a medical device company can integrate Tridion with an eQMS (electronic Quality Management System) to pull in approved labeling content or push updates for regulatory submission packages. This kind of integration ensures that documentation is not an island but part of the broader digital thread of product information.
User-friendly authoring with familiar tools
While DITA XML is structured, authors don’t need to manually tag content or struggle with raw code. Tridion Docs offers a web-based Word-like authoring experience that feels akin to using a regular word processor, with the structure enforced behind the scenes so occasional contributors can start creating content in minutes. This lowers the learning curve that is sometimes associated with DITA, keeps training costs low also reduces onboarding time for interns, contract writers, leading to accelerated adoption of change.
Content creators and SMEs get the best of both worlds: the rigor of XML and the ease of WYSIWYG editing. Every persona, from field engineer to senior tech writer, works in the environment that suits them best, yet the organization maintains one governed content hub.
For power users, Tridion Docs connects seamlessly to professional XML editors such as Oxygen or XMetal via WebDAV or REST API. Features like component metadata, taxonomy tools, variant management, and review workflows are built into Tridion Docs, making it a one-stop solution for managing your entire content lifecycle.
In short, Tridion Docs turns business-critical information into intelligent content by combining the power of DITA with enterprise-grade content management. For organizations, that means reduced risk (thanks to better compliance and accuracy), boosted team productivity, and ultimately better content experiences for end users.
Innovating Content Delivery
Semantic AI-powered Tridion Docs Genius and Trustable Chat
Adopting structured authoring addresses the creation and management of content; but what about delivering that content to end users in the most effective way? Today’s audiences (be they internal employees, field engineers, or customers) expect to find precise answers quickly, not sift through entire PDFs or portals manually.
Recognizing this need, RWS introduced Tridion Docs Genius, an innovative content delivery module, and Trustable Chat, a built-in conversational AI assistant. Together, these innovations elevate structured content from static documents to an interactive knowledge experience.
Tridion Docs Genius Where Content Really Clicks
Tridion Docs Genius introduces a novel ‘knowledge portal’ that presents users with intelligent content recommendations in context. With Hexahops™, hexagon-shaped suggestions guide users through related topics visually, helping them navigate complex information faster (e.g., a field technician finding advanced troubleshooting steps, firmware update procedures, and safety warnings all linked around the task at hand). Tridion Docs Genius Where Content Really Clicks Tridion Docs Genius introduces a novel ‘knowledge portal’ that presents users with intelligent content recommendations in context. With Hexahops™, hexagon-shaped suggestions guide users through related topics visually, helping them navigate complex information faster (e.g., a field technician finding advanced troubleshooting steps, firmware update procedures, and safety warnings all linked around the task at hand).
Traditional content delivery portals often leave users drowning in search results or lengthy manuals. Tridion Docs Genius rethinks this experience by offering a dynamic, AI-powered knowledge portal on top of your structured content repository. Instead of a linear search that returns a list of links, Genius provides three standout capabilities that transform static documentation into guided, contextual answers.
Hexahops™ Visual Navigation
Imagine searching for how to perform a complex equipment setup. Rather than one monolithic page, Genius displays the six most relevant content pieces around your query, each in a hexagon tile – like a honeycomb of answers. This “hop” approach lets you intuitively branch into related sub-topics or procedures, guided by the system’s understanding of your goal. It’s a bit like having a smart guide who not only finds what you asked for but also says “you might need this piece of info next.” This approach drastically reduces the time to assemble information from multiple sources. No more dead-end searches or hunting through PDFs; the system surfaces the connections for you. Hexahops are more than just suggestions. They unlock a treasure trove of in-context recommendations, weaving a path through relevant content.

Contextual Content Delivery
Because Genius sits on top of the knowledge portal, it can pull in exact fragments of content needed. For example, if a field service engineer is trying to replace a component, Genius can present the specific calibration procedure, the safety precautions, and the relevant part specifications side-by-side, each pulled from different approved topics. This contextual mashup is possible only because the content is structured (in DITA) and semantically rich. The result? Users can synthesize information from multiple sections without manually opening multiple documents. This is especially powerful in regulated environments where following the correct sequence and considering all warnings can business critical, or indeed lifesaving.
Trustable Chat: A Reliable AI Assistant, Powered by Your Content
Chatbots and AI assistants are everywhere, but a common gripe is that they often “hallucinate”. They give plausible sounding yet incorrect answers, especially if based on generic LLMs that have been trained on web data. In high-stakes domains (like pharma, med-tech, aerospace), accuracy isn’t negotiable. This is why Tridion Doc’s Trustable Chat is a game-changer: it’s a generative AI interface that only draws from your organization’s verified content in generated from your Tridion Docs repository, using semantic search and retrieval augmented generation (RAG) to ensure answers are grounded in truth.
The built in chatbot only references approved components, and responses include a citation back to the source. Users get instant, verifiable answers without misinformation. In addition, links to the source topics that provided the answer are displayed so that you can cross-reference the answers and be assured they “bot” has not invented the answers.
With Trustable Chat, even a novice user can ask complex procedural questions in plain language (e.g., “Please help me set up the fryer and which ingredients to use”) and receive step-by-step guidance drawn directly from approved content, complete with references. The chatbot provides clear answers and follow-up links, ensuring no misinformation – every response is backed by the source content.
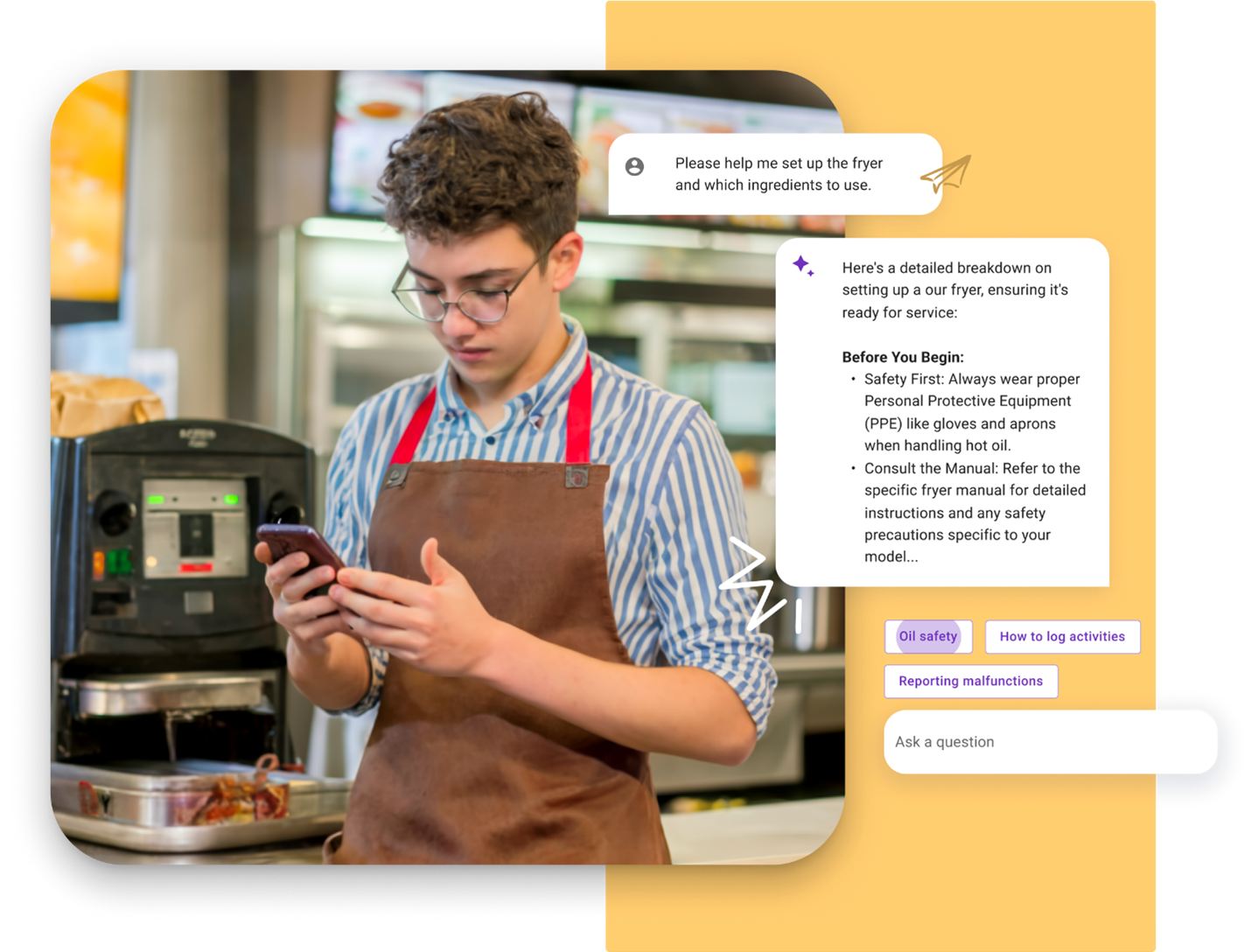
Key features and benefits of Trustable Chat include
Verified Answers Only
The system’s AI “brain” is connected to your CCMS content. It will not invent new information beyond what’s in the manuals, knowledge articles, or documentation you’ve fed it. RWS’s Semantic AI technology digs into your trusted sources to find the truth, so “no fake answers!” For users, this means they can ask questions in natural language and get an immediate, reliable answer in plain English (or any language your content is in), rather than wading through search results. If more details are needed, every answer comes with a reference link to the original document or topic for validation.
Transparency and Trust
Every response from Trustable Chat includes citations – it shows you exactly where it found the information. This builds trust with users (and with your compliance officers!) A technician can see that the answer about a calibration step came from the official Service Manual, revision 3, page 45, for example. This level of transparency is critical in regulated fields: it’s not enough for an AI to give an answer; you need to prove that answer is correct and up-to-date. Trustable Chat provides that audit trail by design.
Efficiency and Empowerment
From a business perspective, having a “virtual expert” available 24/7 to your employees or customers can save enormous support costs and time. New hires can get up to speed faster by asking the chatbot how to perform tasks rather than hunting through training binders. Field service engineers can troubleshoot on the fly, possibly resolving issues without escalations. All this drives productivity.
Alex Abey, GM of RWS content management portfolio, noted that failing to find needed info is not just frustrating, it wastes time and resources, and these new AI features “mark a massive leap forward for content-heavy organizations, who can now open up their information in a visually intuitive and efficient way using the power of AI; without losing confidence in the content.”
In other words, Tridion’s AI actually augments human users instead of misleading them, striking the right balance between convenience and accuracy.
Secure, enterprise-ready AI
It’s worth noting for the IT audience that Trustable Chat’s AI operates within your enterprise content domain. Your sensitive product information isn’t sent to some public API without control; RWS’s approach to “trustable AI” means keeping the data secure and the AI’s scope limited to your content. This ensures compliance with data privacy and regulatory requirements, a must for pharma and other industries dealing with confidential information.
In essence, Tridion Docs Genius and Trustable Chat turn your documentation into an interactive knowledge hub. Organizations appreciate how this improves customer satisfaction (users get what they need faster) and reduces support load, while end-users and technical staff gain a tool that makes their jobs easier. It’s a prime example of how investing in modern content technology can yield returns both in business efficiency and end-user experience.
Real-world results for your business
Seeing is believing, from faster product launches to dramatic cost savings, adopting a new content platform is an investment, and business leaders rightly demand to see the return. Fortunately, with Tridion Docs, the ROI story is compelling, backed by real customer success. Here are some concrete results organizations have achieved by moving to DITA-based structured authoring with Tridion:
Faster time-to-market and streamlined publishing
Informatica, a global software company, transitioned from traditional book-based writing to DITA and Tridion Docs to speed up documentation delivery. The result? In just seven months, they achieved measurable ROI, significantly reducing the time to release product documentation to market. By eliminating laborious desktop publishing steps, they went from staggered, language-by-language releases to true simultaneous global releases. One major project involved producing 10,000 pages in English and Portuguese in under seven months – something previously unimaginable.
“Tridion Docs will pay for itself. By eliminating all of the desktop publishing, we are saving nearly $6 per page – a significant savings when you’re looking at 10,000 plus pages per language."
Martin Levy, Informatica’s Director of R&D Globalization
That translates to around $60,000 saved per language per large doc set just in DTP cost avoidance. Moreover, having content in structured format allowed Informatica to wipe out $40,000–$50,000 in costs per publication project and gain complete in-house control of their documentation process.
Cost reduction through content reuse & automation
The power of “create once, reuse everywhere” directly impacts the bottom line by reducing duplicate work. For example, HORIBA Medical cut translation costs in half after implementing Tridion Docs. How? They maximized content reuse across documents and automated formatting, so the expensive DTP stage for each translated manual was eliminated.
Likewise, Waters Corporation achieved approximately 60% content reuse across their documentation portfolio. This level of reuse means fewer new words to write and translate with each product update, yielding year-on-year cost efficiencies. It also accelerates update cycles (since 60% of the content was already vetted and only 40% needed new work).
Another customer, Nielsen, improved documentation time and cost efficiency “year on year” by leveraging Tridion technology, indicating sustainable ROI growth rather than a one-off gain.
Productivity and headcount impact
A strong ROI case for enterprise CCMS is often the ability to do more with the same team. Philips Image Guided Therapy, as noted earlier, managed to translate increasing volumes of content without increasing their documentation or localization staff. United Imaging, a medical imaging company, found that moving from unstructured docs (in Word files) to structured content eliminated so much duplicated effort that it significantly increased their process efficiency with the same team size. By freeing writers from the tedium of re-writing and layout tweaking, they can focus on creating quality content. One manager quipped that after Tridion Docs, their small documentation team felt like it had gained a few “invisible team members” – the automation and reuse acted like extra help to handle the growth in content demands.
Quality, compliance and risk mitigation
The ROI of structured content isn’t just measured in speed or cost; quality improvements have financial value by reducing errors and compliance risks. For instance, by enforcing consistent content and providing full audit trails, companies avoid costly mistakes like publishing out-of-date instructions or inconsistent safety information.
Waters Corp., an established leader in life‑sciences and analytical instruments, cited improved consistency and compliance as a key benefit of adopting Tridion Docs a critical win in an industry where regulatory scrutiny leaves no margin for error. Fewer errors mean less risk of regulatory penalties or product recalls due to documentation issues – avoiding those scenarios can save potentially millions, though such “soft ROI” is harder to quantify. Still, it’s a critical part of the business case in life sciences and other regulated fields.
Employee onboarding and knowledge retention
Although harder to measure in pure dollars, organizations also see faster onboarding of new writers and SMEs when using a structured approach. Instead of spending months learning the ins-and-outs of hundreds of pages, new team members can quickly assemble needed info by searching the CCMS or using the Genius portal. And the content itself, being modular and topic-based, is easier to digest and update. This translates to saved time and quicker ramp-up, contributing indirectly to ROI by getting products documented sooner and teams operating at full productivity faster.
Many of these benefits are captured in an ROI white paper, developed in partnership with leading industry analysts, which found that a CCMS like Tridion Docs provides “substantial benefits that can make a big difference to your company’s top and bottom line.”
Download the white paper here: Quantifying the benefits and strong ROI of Tridion Docs.
In summary, the investment in structured content pays off through cost savings, faster delivery, better quality, and scalable content operations. When pitching to executives, it’s powerful to combine these hard numbers with strategic benefits like improved customer satisfaction or enabling new digital initiatives (e.g. delivering content to a web portal or chatbot, which can open up new markets and service models).
Use case spotlight: medical devices, pharma, and regulated industries
While structured authoring adds value in any field (software, manufacturing, finance, etc.), it’s often mission-critical in regulated industries. Here’s how Tridion Docs and its ecosystem shine in some of these contexts.
Medical Devices
Documentation for medical devices (user manuals, Instructions for Use, regulatory submissions like technical files) must be accurate, consistent, and compliant with standards like EU MDR (Medical Device Regulation) and FDA requirements. Tridion Docs helps med-tech companies create a single source of approved content that can populate all their outputs, from print Instructions for Use (IFU)s to digital help and regulatory documents, ensuring that each piece of information (a warning, a procedure step, a spec) is identical wherever it appears. This is crucial when a change (say a new contraindication or updated part number) must propagate to dozens of documents in multiple languages.
With Tridion, companies can update the module once, and publish updated content everywhere with confidence. The result is not only compliance but also speed: being able to update and re-publish global documentation quickly means devices get to market faster and stay in market with current info.
Several medical device firms have publicized improvements after adopting Tridion Docs. For example, Philips reduced translation costs and automated content workflows, translating more content with the same staff and reusing content for new purposes like education material. HORIBA Medical scaled their small documentation team to handle triple the product lines by switching from Word files to Tridion’s structured content (seven writers now maintain content that previously would overwhelm them). Waters Corporation (life science lab equipment) ensured regulatory compliance and consistency across thousands of pages by standardizing on Tridion Docs, and can now implement content changes much more rapidly than before.
Pharmaceuticals and Life Sciences
Pharma companies historically have huge volumes of content, from R&D documentation to manufacturing procedures to marketing and medical information, often created in unstructured formats (Word, PowerPoint, etc.) and stored in disparate systems. This fragmentation is now a liability, as the industry moves toward omnichannel communication and strict compliance.
Structured content is emerging as a solution to unify and efficiently reuse information across departments. Tridion has been working with leading pharma enterprises (including names like Eli Lilly, Sanofi, and Abbott) to enable this omnichannel transformation. For example, a single source of approved content about a drug can be used in physician educational materials, health authority submissions, marketing websites, and internal training, all tailored as needed but kept consistent. One case study presented by a partner showed that implementing a structured content and reuse solution in a pharma company led to “significant savings and enabled ease of use” for teams collaborating across countries. Regulatory affairs groups are particularly interested in how structured content (potentially aligned with standards like IDMP or SPL for labeling) can make assembling dossiers faster and less error-prone. And with Tridion’s metadata and taxonomy capabilities, pharma companies can better classify and retrieve content (e.g., find all docs related to a particular ingredient or study), a huge win for both compliance audits and content personalization downstream.
Simply put, structured content management in pharma is both a compliance safeguard and a backbone for modern, digital-first communication strategies. Tridion’s ability to support multilingual, multi-audience content from one source is especially valuable in this sector, where information needs to be consistent globally but delivered locally.
Other Regulated Sectors (Finance, Government, Aerospace)
Any industry facing strict documentation requirements and oversight can benefit from the approach we’ve outlined. In finance and insurance, for instance, using a CCMS can ensure that customer-facing documents (policies, disclosures) always use the latest approved wording and legal clauses, reducing risk of non-compliance.
Government agencies dealing with standards or large policy manuals can use structured authoring to keep content updated and accessible to the public in multiple formats (web, print, accessible formats) as required by law. Aerospace and defense, as discussed, often mandate S1000D for certain deliverables, RWS has solutions, like Contenta, for that as well, but many are supplementing with DITA-based content for things like training materials or customer support content, where DITA’s flexibility and the advanced delivery options (like Genius portal) offer an improved experience. The common thread is that these industries value accuracy, accountability, and efficiency – exactly the strengths of Tridion Docs and structured content.
Finally, a regulated industry angle that shouldn’t be overlooked: audit and accountability. With Tridion, every piece of content has an owner, a history, and linkages to where it’s used. When an auditor asks “where is this information documented?” or “prove that all your user manuals include the latest safety warning,” you can generate a report or trace in minutes. That kind of agility under scrutiny is itself a significant ROI for companies frequently audited or certified.
Conclusion: unlocking your knowledge
It’s time to think of content not as a collection of static documents, but as core business assets that can be created, managed, and delivered in smarter ways. Embracing DITA was the first step toward modular, reusable content. Now, leveraging that foundation through an enterprise solution like Tridion Docs elevates the benefits to a whole new level. You achieve the control and consistency required by regulated industries, and the agility and innovation (AI-driven delivery, omnichannel experiences) that modern digital transformation promises.
From a business executive’s perspective, structured content delivers tangible ROI – faster time to market, lower costs, improved quality, and happier customers. It also future-proofs your content operations in an era where information volume is exploding and personalization is key.
From a hands-on user’s perspective, it means no more wrestling with format issues or copying and pasting updates across dozens of files; instead, you focus on crafting great content and let the system take care of reuse, formatting, and publishing. And with tools like Genius and Trustable Chat, your hard work as a technical communicator is even more impactful – it reaches the end user in the most accessible form possible, right when they need it.
DITA-based structured authoring with Tridion Docs is more than a superior choice; it’s rapidly becoming the expected standard for organizations that value efficiency, accuracy, and engagement.
Whether you’re producing surgical robot manuals, global pharma guidelines, or complex software documentation, the combination of DITA/XML structure, a robust CCMS, and cutting-edge delivery tech is a winning formula. It empowers your team to deliver the right content, at the right time, in the right format, all while maintaining control and ensuring compliance.
That’s a message worth embracing.
Ready to take the next step? Reach out to RWS for a demo of Tridion Docs and see how structured content can transform your documentation workflow.
Your content is a goldmine – with the right approach, you can unlock its full potential.
