Accelerating Speed to Market: How Structured Content Drives Competitive Advantage

Why documentation speed defines success
In large enterprises, outdated document workflows can significantly stall product launches, delay strategic initiatives, and inflate operational costs. For regulated industries such as pharmaceuticals, finance, or aerospace, documentation delays aren't just costly, they are non-negotiable. The stringent standards in regulated industries provide a clear model of best practice that any enterprise can adopt to achieve faster, more efficient content management. Regulatory clocks never pause, and for example in MedTech, while R&D races to innovate, product launches often stall because documentation, labeling, and clinical evidence run through outdated document workflows.
In all cases, every extra review cycle increases costs, reduces revenue, and, in some cases, can have a profound impact on the end-users' lives.
Structured content turns that chokepoint into a competitive edge for small, medium and large global enterprises. By breaking information into reusable, metadata-rich components inside a Component Content Management System (CCMS), teams update once and publish everywhere, simultaneously, in every language, with full audit traceability.
The result
Compressed timelines, lower translation spend, and smoother approvals. This post builds on our "A practical guide to structured content migration" and dives into one outcome businesses care about most: speed-to-market for medical devices. Let’s look at the pain, the fix, and the proof.
Medical device companies share three non-negotiables
- Speed: hit launch dates before the competition
- Compliance: satisfy regulators from Brussels to Brasília
- Cost control: do more with the same headcount
Together, these demands make documentation speed mission-critical.
Enterprise content-workflows weren’t built for speed, or scale
Many enterprises still rely on outdated document workflows; using large binary files like PDFs, Word documents, and InDesign layouts. This approach creates costly inefficiencies, including:
- Manual copy-and-paste updates across multiple documents, increasing the likelihood of errors and inconsistencies.
- Managing dozens of nearly identical document variations individually, resulting in wasted time after each minor change.
- Repeated translation of nearly identical content for every language version, inflating costs and delaying market entry.
- Desktop publishing bottlenecks that can add days or even weeks to routine content revisions.
- Quality assurance teams forced to revalidate entire documents following minor edits, consuming valuable time and resources.
These issues sharply impact productivity and responsiveness in every enterprise.
For regulated industries such as medical devices still working with massive Instructions for Use (IFU), pharmaceuticals, or aerospace, these inefficiencies become even more critical, introducing compliance risks, costly delays, and barriers to innovation.
Regulated industries highlight these inefficiencies most vividly, but these pain points affect productivity and responsiveness across all sectors. for example, medical device teams still working with massive Instructions for Use (IFU), disconnected tools, and manual checks face costly delays and compliance risks at every turn.
The result: Slow content updates, increased operational costs, and frustrated end-users
The hidden cost of slow documentation is high, not only in missed revenue and opportunity, but in risk exposure if content isn’t up-to-date and compliant.
With the competitive landscape of organisations racing to innovate, enterprises that streamline their content operations gain a clear edge. Innovators that nail all three, speed, compliance, and cost control, win big.
The fastest path? Structured Content Authoring.
Large enterprises adopting structured content report substantial business gains:
- Reduced translation expenses by reusing content across languages and markets, typically achieving 30–50% savings
- Faster content updates, with document publishing reduced from weeks to just hours
- Significant operational cost savings, often exceeding 60%, through streamlined workflows and reduced duplication
Regulated industries offer clear examples:
- Pharmaceutical companies have accelerated documentation timelines by as much as 70%
- Financial institutions have shortened policy update cycles dramatically by adopting structured content practices
- Aerospace manufacturers consistently achieve high content reuse rates, greatly cutting costs
Speedy, efficient documentation is no longer a “nice-to-have” – it’s a strategic necessity for compliance and competitive advantage and essential for businesses that intend to lead rather than follow.
Structured Content Authoring Explained
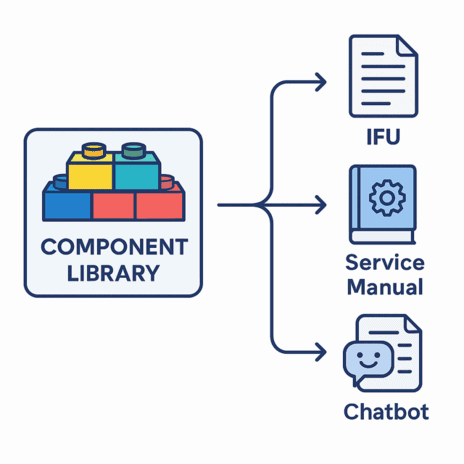
Structured content authoring (SCA) is a methodology for creating content in modular, reusable chunks (components) like paragraphs, safety notifications, or procedures), that are stored and managed in a central repository. Instead of treating documents as standalone files, teams contribute content components once and then make them available for reuse across all necessary documents.
SCA captures knowledge as reusable, semantically tagged components (topics, graphics, data modules). One approved component can populate any output type: IFU, service manual, chatbot, video script.
So what is DITA?
DITA (Darwin Information Typing Architecture) is an open XML standard for structured content. DITA was originally developed by IBM and later donated to OASIS (a standards organization) in 2005, which means it’s a vendor-neutral and tool-independent XML standard.
In simpler terms, DITA is a set of rules and a framework for writing content in a consistent, modular way. DITA defines content in topics (such as concepts, tasks, and reference topics) rather than large documents. It encourages writing content as small, self-contained topics that can be mixed, matched, and reused in different deliverables.
Many CCMS and authoring tools support DITA out-of-the-box, making it a popular choice for implementing structured content.
DITA, simplified
- XML at its core: Uses machine-readable markup, easily parsed by any modern authoring tool or component content management system (CCMS). XML uses tags to label content semantically (for example, identifying something as a “<step>
Procedure Step</step>” or “<hazard>Note</hazard>” rather than just making text bold or italic). - Topic-based authoring: Instead of unwieldy documents, writers craft small, standalone topics; like concepts, tasks, or references, that are easy to manage and reuse.
- Designed for structure: Content hierarchy and relationships are clearly defined in maps, making reuse straightforward.
- Separate content from formatting: Content styling happens through style sheets during publishing, allowing the same source to instantly transform into PDFs, web pages, mobile content, or even chatbot interactions.
- One source, endless possibilities: Translate or adapt content once, then effortlessly publish it across multiple formats and languages without ever revisiting the original.
But how does structured content work?
- Component content: Capture every warning, procedure, or graphic once as a tagged component. One edit updates IFU, eIFU, service, and training content in parallel.
- Rich metadata: DITA-based specialisations label each piece with device class, risk level, language, and audience, making it machine-readable for AI and traceability.
- Real-time validation & e-signatures: Built-in rules stop non-compliant content at save time, not at the final PDF, and electronic signatures provide proof of approval and auditable trails.
- Single-click publishing: The CCMS outputs PDF, HTML5, EPUB, JSON, eSTAR, or pushes to OEM apps, so that regulators, technicians, and chatbots all get the latest version instantly.
- Immutable audit history: Every change, approval, and rollout is logged automatically, giving QA a full, regulator-ready trail in seconds.
The result
Faster approvals, lower translation spend, and zero copy-paste errors; exactly what medical-device launches need to hit market dates.
Key benefits of structured content for speed-to-market
Regulatory hot buttons and SCA solutions
| Compliance driver (industry) | Typical pressure points | How structured content helps |
| Life Sciences & MedTech (EU MDR / IVDR, FDA 21 CFR 820 + Part 11) | Clinical-evidence ramp-up, e-labelling, UDI submissions, electronic records, cybersecurity SBOM |
Single-source components feed both PDF submissions and digital portals; e-signatures & audit trails satisfy regulators with minimal rework. |
| Financial Services & Insurance (SEC / FINRA / ESMA) | Rapid rule updates, disclosure consistency, auditability of client communications | Reusable, metadata-rich components auto-populate all disclosures and statements; traceability proves compliance and speeds reviews. |
| Aerospace & Automotive (AS9100, ISO 26262) | Safety-critical documentation, multi-variant product lines, frequent design updates | Component reuse ensures identical safety language across manuals, service bulletins, and training; version history accelerates sign-off. |
| Energy & Utilities (NERC CIP, ISO 55001) | Operational safety procedures, multi-jurisdiction approvals, emergency update cycles | Central repository pushes validated content instantly to field devices, web portals, and regulators; minimizes downtime during audits. |
| Data Privacy & InfoSec (GDPR, CCPA, ISO 27001) | Rapid policy changes, multi-language notices, proof of consent | Modular content allows quick, uniform updates across web, mobile, and contracts; audit trails demonstrate consent-record management. |
| Enterprise Best Practice (cross-industry) | Brand consistency, legal accuracy, M&A integration, ESG reporting | One “source of truth” eliminates duplicate authoring, speeds localization, and delivers consistent messaging to all stakeholders. |
Non-compliance can halt shipments, trigger fines, or erode trust overnight. Structured content compresses update cycles, reduces translation spend, and provides the transparent audit trail regulators —and boards—demand. Even in less-regulated sectors, adopting these practices elevates quality, accelerates time-to-market, and lowers risk.
Single-source updates
Edit content once and it's updated universally, avoiding the slow, error-prone task of hunting through dozens of files for manual updates. For instance, pharmaceutical companies have reduced their document creation timelines from 12 weeks to just 2 by adopting reusable components.
High content reuse across product families
Medical device manufacturers frequently produce content for product families or model variations. Structured content allows a single piece of core content, like a standard procedure or specification, to be updated instantly across all those product/model variations, achieving reuse rates of up to 60%, significantly cutting costs and speeding processes. A global life sciences leader, for instance, achieved up to 60% content reuse in their documentation library, saving significant time and cost. Higher reuse means writers spend less time duplicating effort and more time on new, value-added content.
Parallel authoring and collaborative review
Traditional sequential reviews become concurrent collaborations. With a component content management system (CCMS), multiple authors, editors, and subject matter experts can collaborate simultaneously on different pieces of content. Instead of a linear review cycle (where one person waits for another to finish), stakeholders can contribute in parallel on a cloud-based platform. Edits and comments are visible in real time to the whole team. This concurrent collaboration collapses review timelines dramatically – no more emailed Word docs or PDF markups drifting out of sync. Everyone works on the latest version in a single source, which speeds up approvals and ensures nothing slips through the cracks.
Central governance and audit trails
All content components live in one central, managed location. This centralization is transformational for complex medtech documentation pipelines. Instead of content being scattered across local files and department silos, everything resides in one governed system. This prevents vital information from being lost and ensures audit trails and version control on every component, gold for regulatory compliance, and for organizational peace of mind and confidence. Digitizing and centralizing reduce compliance risks significantly. Teams create, review, approve, and distribute content through a unified online platform.
Faster, cheaper localization
Structured content also excels in localization. Translate a component once, reuse it everywhere needed, eliminating repetitive translation tasks and drastically reducing global rollout times. Medtech products are often global or have a goal to be, and translating documentation is traditionally a huge source of delay. Because content is componentized, you only translate a piece of content once and then reuse that translated component in all relevant documents. There’s no need to re-translate full documents for each market. This means localization happens quicker and at lower cost, trimming weeks off the launch schedule for additional geographies. Organizations can even parallelize translation work and start it earlier (translating components as they are finalized rather than waiting for an entire document), further speeding time-to-market in multiple regions.
From change request to global update in minutes: how does SCA accelerate market entry
One example of where the reuse of content proves its worth is where a change has occurred due to a change in design and the documentation department can write once, update once, publish everywhere.
How it works in a medical-device workflow
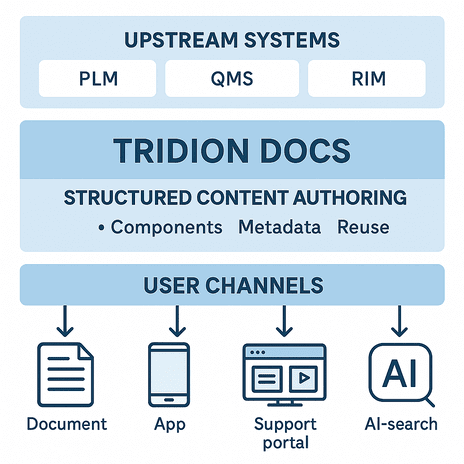
Scenario
Your defibrillator’s user manual, service guide, and eIFU all contain the same electrical-shock warning:
DANGER — Risk of electric shock. Disconnect power before servicing.
Engineering introduces a hardware safeguard that lowers the risk from danger to caution.
Here’s how structured content handles the change:
|
Step |
What happens in the CCMS |
What your teams (and regulators) see |
|
1 . Capture once |
The warning is stored as a single, tagged component:
|
Writers, trainers, translators all reference one source, not multiple Word files. |
|
2 . Reuse everywhere |
The CCMS automatically pulls that component into every output that needs it: PDF IFU, service manual, HTML5 eIFU portal, eSTAR submission tool, technician chatbot, and 23 language variants. |
Consistent wording and iconography across all channels. |
|
3 . Update once |
A technical author edits the component text to: CAUTION — Risk of electric shock. Disconnect power before servicing. (Metadata flag changes from risk=high to medium). |
Immediately, every linked document shows CAUTION instead of DANGER. No copy-paste, no hunting through files. |
|
4 . Auto-validation & audit |
Save triggers the required electronic signatures, schema validation, and a timestamped audit entry. |
QA downloads an inspection-ready report proving who changed what, when, and why. |
|
5 . Instant output |
One publish action regenerates PDFs, HTML5, EPUB, eSTAR compatible files, JSON, and translation bundles. |
Field service, customers, and auditors all get the latest, compliant content within minutes. |
The payoff
- Regulatory confidence: Single change logs across all languages and outputs
- Time saved: Weeks of manual edits collapse into minutes
- Translation cost down: Only the 12 words that changed are re-translated, not entire manuals
- Launch on schedule: Documentation never holds up 510(k), MDR, or IVDR submissions
Real-world impacts: Speed and Efficiency Gains
The impact of structured content authoring time-to-market isn’t theoretical; it’s proven in highly regulated industries.
Pharmaceutical companies have reported cutting document creation time by up to 70% by switching to structured content and leveraging automation. Novo Nordisk, a global pharma leader, achieved a 70% reduction in documentation timelines after overhauling their process with structured content and component reuse. In practical terms, what used to take three months of writing and review now takes only a few weeks.
One medical device documentation team cited how shifting to a component content management strategy turned their content process “from a potential bottleneck into a strategic advantage”. They could respond to a compliance change or a product tweak in a fraction of the time it used to take, confident that all outputs would be updated consistently overnight.
“We reduced translation costs by 60% and translation time by 40%. And my team’s ‘happiness index’ increased by 80%.”
Emily Mydlowski, Technical Publications Manager, Hach Company
Case studies
- HORIBA Medical maximizes content reuse and cuts translation costs in half with Tridion Docs
- Philips streamlines documentation processes and saves translation costs
- Waters Corporation saves time and cost with up to 60% content reuse
- United Imaging Healthcare eliminates duplication of effort with Tridion Docs
The sooner you break free from copy-paste document workflows, the sooner you harvest the time savings. In regulated industries, those saved weeks or months can translate directly into market advantage and revenue.
Business Outcomes: Compliance, Efficiency, and Competitive Edge
Beyond raw speed, structured content delivers tangible business outcomes
Enhanced messaging, compliance, and risk management
Structured content enforces consistent terminology, messaging, and critical information across all enterprise documentation. In regulated sectors like pharmaceuticals, finance, and aerospace, this level of precision is non-negotiable. But for any large enterprise, adopting these best practices drives measurable gains: increased accuracy, reduced risk, and greater operational efficiency.
Validated content components and embedded audit trails reduce rework, streamline reviews, accelerate approvals, and reinforce stakeholder confidence in your organization’s governance and control. This builds trust with stakeholders by demonstrating a transparent documentation process.
Greater efficiency and cost savings
Efficiency improvements go hand-in-hand with speed. Automated content assembly frees teams from manual tasks, allowing focus on high-value content creation and accomplish more in less time (often with the same or fewer resources). Maintenance becomes easier – if a new regulation or product change comes along, you update a handful of components instead of hundreds of documents. This agility ensures documentation is no longer a bottleneck for the business.
Competitive advantage
Structured content doesn’t merely streamline documentation, it transforms enterprise agility. Faster, more flexible content processes enable large enterprises across all sectors to launch products quicker, respond swiftly to market shifts, and outperform competitors. The rigorous standards that regulated industries adhere to become best practices for any company seeking robust, scalable, and future-proof documentation strategies.
Just as importantly, structured content prepares organizations for the future. It enables new capabilities like content personalization, easier updates for new markets, and integration with emerging technologies (e.g. AI-driven content assistance or chatbots). Early adopters in structured content are effectively future-proofing their content operations. Companies that cling to old methods accumulate “content debt” – hidden inefficiencies that make them slower to respond and innovate.
In the race to medical device innovation, those with streamlined content engines will outpace those on horseback. In regulated MedTech, the cost of doing nothing grows every day, while those who invest in the advantages only structured content can guarantee are already reaping the rewards and delighting stakeholders.
The payoff is clear: faster approvals, lower costs, and a stronger market position
Structured content is transforming medical device documentation from an obstacle into an advantage.
By enabling simultaneous updates, widespread content reuse, and centralized collaboration, SCA drastically reduces time-to-market for documentation without sacrificing quality or compliance. Documents that once were static burdens now become dynamic strategic assets, fueling faster product launches and more agile updates.
Are you ready to turn your enterprise content from a bottleneck into a strategic advantage? Download our white paper “When Documents No Longer Cut It” to find out how structured content can streamline your documentation processes, enhance organizational agility, and deliver tangible business value.
Then book a call to talk with an RWS strategist to understand how best practices from regulated industries can help your enterprise thrive and start mapping your path to a CCMS.
This is the fourth in our ten-part series on mastering the shift from unstructured to structured content. We unpack the practical and mindset approaching towards successful implementations of structured content authoring and a CCMS.
- Part one: If it's not broken, why fix it?
- Part two: A practical guide to structured content migration and streamlining content operations
- Part three: Trapped in the Document Paradigm
Look out for blog five, coming soon.
